Difference between revisions of "HFE"
(→Protein Sequence) |
|||
| Line 1: | Line 1: | ||
| − | HFE is named after '''H'''igh '''Fe''' (iron). The gene products known function is to regulate iron absorption by interacting with the transferrin receptor (TfR). When the normal function is disrupted by mutation the result can be HFE hereditary [[hemochromatosis]] or iron overload. | + | HFE is named after '''H'''igh '''Fe''' (iron). The gene products known function is to regulate iron absorption by interacting with the transferrin receptor (TfR). Both HFE and transferrin (Tf) compete with each other for binding to the receptor (TfR)<ref>Lebrón, J. A., West Jr, A. P., & Bjorkman, P. J. (1999). The hemochromatosis protein HFE competes with transferrin for binding to the transferrin receptor. Journal of molecular biology, 294(1), 239-245.</ref>. Tf binds to iron in the form of diferric transferrin (Fe-Tf) and binds to free TfR which takes the iron into the cell by an endocytosis cycle<ref>Qian, Z. M., Li, H., Sun, H., & Ho, K. (2002). Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacological reviews, 54(4), 561-587.</ref>. When the normal function of HFE is disrupted by mutation the result can be HFE hereditary [[hemochromatosis]] or iron overload. |
=DNA Sequence= | =DNA Sequence= | ||
Revision as of 10:20, 18 July 2014
HFE is named after High Fe (iron). The gene products known function is to regulate iron absorption by interacting with the transferrin receptor (TfR). Both HFE and transferrin (Tf) compete with each other for binding to the receptor (TfR)[1]. Tf binds to iron in the form of diferric transferrin (Fe-Tf) and binds to free TfR which takes the iron into the cell by an endocytosis cycle[2]. When the normal function of HFE is disrupted by mutation the result can be HFE hereditary hemochromatosis or iron overload.
DNA Sequence
HFE is located on chromosome 6 in humans.
RNA Sequence
There are several alternatively spliced variants of HFE.
Protein Sequence
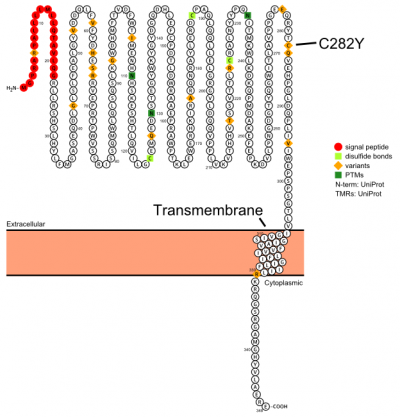
The protein has a signal sequence and transmembrane domain. It forms a tertiary complex with beta2-microglobulin (beta2M) and TfR. HFE has sequence and structural similarity to MHC class I-type proteins.
NCBI Protein[1]
>gi|4504377|ref|NP_000401.1| hereditary hemochromatosis protein isoform 1 precursor [Homo sapiens] MGPRARPALLLLMLLQTAVLQGRLLRSHSLHYLFMGASEQDLGLSLFEALGYVDDQLFVFYDHESRRVEP RTPWVSSRISSQMWLQLSQSLKGWDHMFTVDFWTIMENHNHSKESHTLQVILGCEMQEDNSTEGYWKYGY DGQDHLEFCPDTLDWRAAEPRAWPTKLEWERHKIRARQNRAYLERDCPAQLQQLLELGRGVLDQQVPPLV KVTHHVTSSVTTLRCRALNYYPQNITMKWLKDKQPMDAKEFEPKDVLPNGDGTYQGWITLAVPPGEEQRY TCQVEHPGLDQPLIVIWEPSPSGTLVIGVISGIAVFVVILFIGILFIILRKRQGSRGAMGHYVLAERE
NCBI Structure[2]
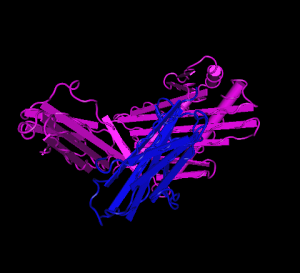
HFE (magenta) tertiary complex with beta2M (blue)[3]
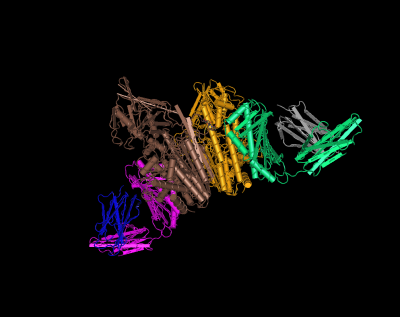
HFE (magenta and green) tertiary complex with beta2M (blue and gray) and TfR (yellow and brown)[4]
Plot in Protter[3] with UniProt accession: Q30201
- ↑ Lebrón, J. A., West Jr, A. P., & Bjorkman, P. J. (1999). The hemochromatosis protein HFE competes with transferrin for binding to the transferrin receptor. Journal of molecular biology, 294(1), 239-245.
- ↑ Qian, Z. M., Li, H., Sun, H., & Ho, K. (2002). Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacological reviews, 54(4), 561-587.
- ↑ Lebron, J. A., Bennett, M. J., Vaughn, D. E., Chirino, A. J., Snow, P. M., Mintier, G. A., ... & Bjorkman, P. J. (1998). Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell, 93(1), 111-123.
- ↑ Bennett, M. J., Lebrón, J. A., & Bjorkman, P. J. (2000). Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature, 403(6765), 46-53.