HFE
HFE is named after High Fe (iron), and unusually is not named after its associated disease hemochromatosis that led to the genes identification. The gene products known function is to regulate iron absorption by interacting with the transferrin receptor (TfR). Both HFE and transferrin (Tf) compete with each other for binding to the receptor (TfR)[1]. Tf binds to iron in the form of diferric transferrin (Fe-Tf or holo-Tf[2]) and in turn binds to free TfR which imports the iron into the cell by an endocytosis cycle[3]. The peptide hormone hepcidin is produced in the liver, increased hepcidin levels inhibit iron uptake by degrading the ferroportin (Fpn) iron exporter to the blood. Unbound HFE in the presence of holo-Tf also induces higher transcription levels of hepcidin via an unclear mechanism[4]. When the normal function of HFE is disrupted by mutation the result can be HFE hereditary hemochromatosis or toxic iron overload.
DNA Sequence
HFE is located on chromosome 6 in humans.
RNA Sequence
There are several alternatively spliced variants of HFE.
Protein Sequence
The protein has a signal sequence and transmembrane domain. It forms a tertiary complex with beta2-microglobulin (beta2M) and TfR. HFE has sequence and structural similarity to MHC class I-type proteins.
NCBI Protein[1]
>gi|4504377|ref|NP_000401.1| hereditary hemochromatosis protein isoform 1 precursor [Homo sapiens] MGPRARPALLLLMLLQTAVLQGRLLRSHSLHYLFMGASEQDLGLSLFEALGYVDDQLFVFYDHESRRVEP RTPWVSSRISSQMWLQLSQSLKGWDHMFTVDFWTIMENHNHSKESHTLQVILGCEMQEDNSTEGYWKYGY DGQDHLEFCPDTLDWRAAEPRAWPTKLEWERHKIRARQNRAYLERDCPAQLQQLLELGRGVLDQQVPPLV KVTHHVTSSVTTLRCRALNYYPQNITMKWLKDKQPMDAKEFEPKDVLPNGDGTYQGWITLAVPPGEEQRY TCQVEHPGLDQPLIVIWEPSPSGTLVIGVISGIAVFVVILFIGILFIILRKRQGSRGAMGHYVLAERE
NCBI Structure[2]
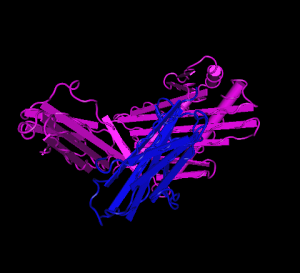
HFE (magenta) tertiary complex with beta2M (blue)[5]
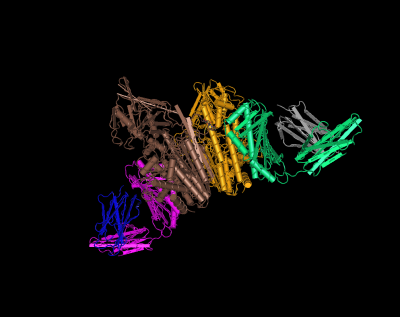
HFE (magenta and green) tertiary complex with beta2M (blue and gray) and TfR (yellow and brown)[6]
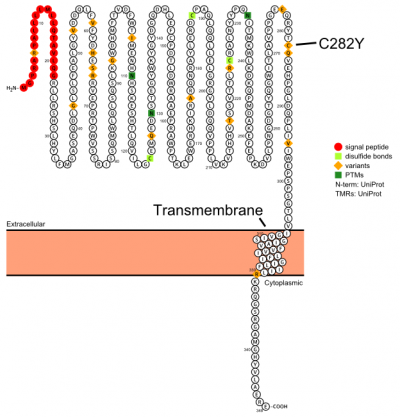
Plot in Protter[3] with UniProt accession: Q30201
To Do
Hentze, M. W., Muckenthaler, M. U., Galy, B., & Camaschella, C. (2010). Two to tango: regulation of Mammalian iron metabolism. Cell, 142(1), 24-38.
Wang, J., & Pantopoulos, K. (2011). Regulation of cellular iron metabolism. Biochem. J, 434, 365-381.
Fleming, R. E., & Ponka, P. (2012). Iron overload in human disease. New England Journal of Medicine, 366(4), 348-359.
- ↑ Lebrón, J. A., West Jr, A. P., & Bjorkman, P. J. (1999). The hemochromatosis protein HFE competes with transferrin for binding to the transferrin receptor. Journal of molecular biology, 294(1), 239-245.
- ↑ The holo- prefix refers to a protein bound to its ligand. apo- is the unbound form of the protein.
- ↑ Qian, Z. M., Li, H., Sun, H., & Ho, K. (2002). Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacological reviews, 54(4), 561-587.
- ↑ Gao, J., Chen, J., Kramer, M., Tsukamoto, H., Zhang, A. S., & Enns, C. A. (2009). Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell metabolism, 9(3), 217-227.
- ↑ Lebron, J. A., Bennett, M. J., Vaughn, D. E., Chirino, A. J., Snow, P. M., Mintier, G. A., ... & Bjorkman, P. J. (1998). Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell, 93(1), 111-123.
- ↑ Bennett, M. J., Lebrón, J. A., & Bjorkman, P. J. (2000). Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature, 403(6765), 46-53.