Here is one of the latest results from fruit fly crosses I am running to select examples for my teaching lab this fall. It results in a striking, if not somewhat disturbing, phenotype; however, it illustrates many important concepts simultaneously and is likely to be an example the students will remember.
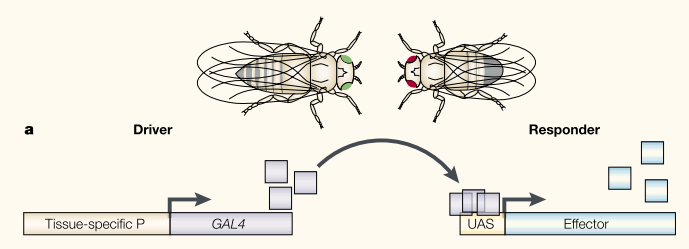
The GAL4/UAS binary expression control system has been an extremely useful tool in Drosophila genetics. The system was developed by Brand and Perrimon (1993). Genes have promoters where transcription begins to express the gene. There are also activator and repressor sequences that can modify gene expression (essentially by turning the gene on and off or, perhaps more appropriate, up and down in an analog scale). This form of gene regulation (transcriptional regulation) is accomplished by the effects of proteins (which are themselves coded by genes) that bind to specific DNA sequences (or to other proteins that are bound to DNA sequences). This begins to bring up the idea of a gene interaction network where genes turn each other on and off, which can quickly become quite complex--perhaps similar to (if it were highly parallel and simultaneous) control flow in computer programming as a metaphor.
In yeast, GAL4 is a protein that forms a dimer (two units bind together) and functions as a transcription activator. It binds to a specific DNA sequence called "UAS" (upstream activation sequence). Yeast "prefers" (i.e. has primarily evolved) to use glucose for energy production (ATP) and reducing power (NADH) in the cells biochemical reactions. However, if there is no glucose and galactose is available GAL4 is produced (glucose represses the GAL4 gene by causing proteins to bind to a URS (upstream repression sequence) and galactose triggers other proteins to bind to a GAL80 protein which also normally suppresses the GAL4 gene) which activates expression of genes used to metabolize galactose by binding to their UAS DNA sites. So in the end we end up with the biochemical logic: IF glucose is not around AND galactose is, the genes for metabolizing galactose are turned on.
If you read all of the details above you should realize this is the tip of the iceberg. Gene interaction networks can be very complex, sometimes non-intuitive, and cannot always be thought of in simple on/off terms. I can't help thinking of the results of biological evolution as Rube-Goldberg machines from time to time, like the one below designed to sharpen pencils.
OK, so if you want to genetically modify Drosophila to do anything interesting you need to express a gene sequence, prevent a gene from being expressed, or change gene expression in some way. But what is the pattern of expression you want to use? It is difficult to redesign different transcriptional regulation sequences and repeatedly transform the flies. You could design the gene to be "on" and produced at a high level all of the time, but what if it is lethal if expressed at some stage of development, etc? Also, this doesn't allow you to study the effects of different expression patterns themselves. On the other hand, it is very easy to cross different fly lines together.
Brand and Perrimon (1993) transformed flies with the GAL4/UAS system from yeast. GAL4/UAS does not exist in flies so in theory it should work independently of the flies own gene regulatory network. Importantly this allowed systems to be divided so a fly line with GAL4 protein being produced with a specific expression pattern can be crossed to a line with a gene under UAS control. This allows GAL4 to drive expression of the gene according to its pattern of transcriptional regulation. Building up a library of different GAL4 lines (using enhancer traps that I will talk about another time) allows a wide range of expression patterns to be tested with a single UAS controlled gene that only has to be created in the lab a single time.
- An illustration of the GAL4 UAS system from Wimmer (2003).
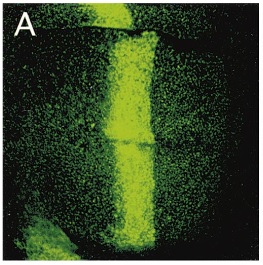
Now let's talk about a gene called decapentaplegic or dpp for short. dpp is expressed in a band through the middle of a structure in Drosophila larvae called imaginal discs. It is a morphogen and acts as one of the signals for specifying the relative position of cells in the imaginal disc during development. Insects like Drosophila go through a metamorphosis from larvae to adults and new adult structures have to be formed like 6 legs, 2 wings, 2 halteres, 1 set of mouthparts, 2 antenna, and 2 eyes. In the larvae these appendages start out as imaginal disks and you can count up 15 of these; thus deca-penta-plegic. In the image of the imaginal disc below (from Teleman and Cohen 2000) GFP (green fluroscent protein) is being expressed in a dpp pattern using the GAL4/UAS system (dpp-GAL4, UAS-GFP).
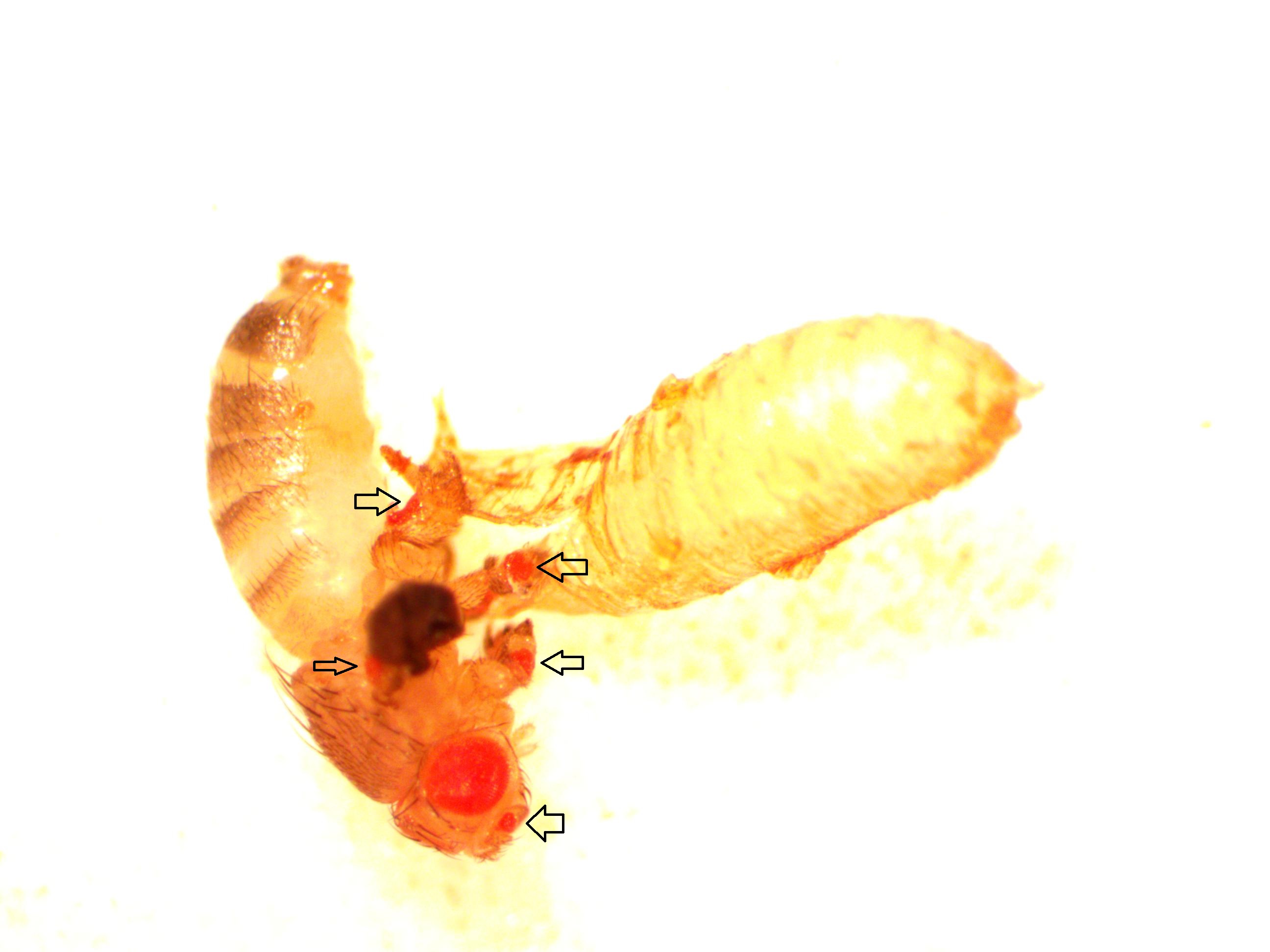
Now let's mention a different gene, eyeless. Drosophila only have four pairs of chromosomes and eyeless is one of the (relatively) rare genes that is on the tiny fourth chromosome, sometimes called the "dot" chromosome. As I mentioned before, the names of genes are kind of confusing. They are often named in a reverse fashion because, in classical genetics, they were only discovered when mutated. eyeless is a master switch that triggers other genes to form eyes. When it is inactivated the flies become eyeless; so if eyeless is functioning correctly the flies are not eyeless. Normally eyeless is only expressed in part of the head. However, if we insert another copy of eyeless into the fly genome under UAS control (I'll talk about how to actually do that in another post) and cross this to a fly with GAL4 expressed with a dpp enhancer, we should trigger eye formation in the other appendages. (A critical unspoken detail is that dpp is expressed early enough in development for eyeless to trigger eye formation. Other sets of drivers may or may not work if the timing is off.)
Above is a male that has just eclosed (emerged from the pupal case). In addition to the normal red eyes you can see small eyes on the antennae, back of the wing (most of the wing is shriveled and dark and above the plane of focus in this image, and on each of the legs. I've pointed them out with arrows below.
I can't help but to think of Argus in Greek mythology.
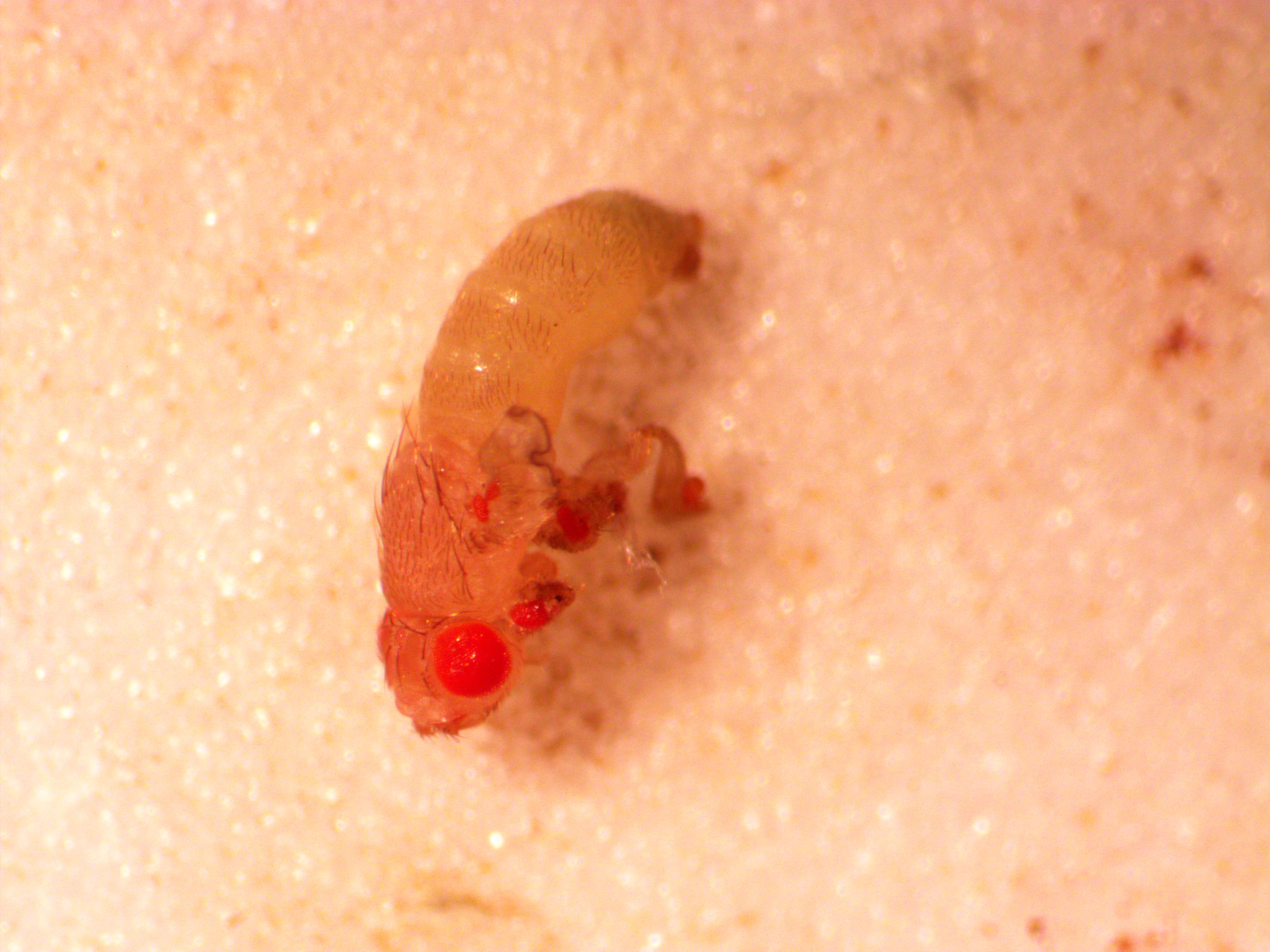
Here is another fly.
And zooming in from above, you might be able to just see facets (ommatidia) on the ectopic eyes.
Here is another that has one leg longer than the others, but still with an eye at the end.
And more of a close up from the other side.
The gene eyeless also exists in vertebrates where it is known as Pax-6. Disruptions in humans result in problems with eye development known as anridia (the iris is missing). Pax-6 is also responsible for eye development in molluscs (octopus, squid, etc.). In fact, the Pax-6 gene sequence from squid, fish or mice can drive ectopic eye formation in Drosophila just like eyeless (Nornes et al. 1998 and references therein). This suggests that the genetic control of eye formation among animals is shared (homologous), very ancient and did not arise multiple times by convergent evolution; and that the differences in eye structures and development among animals evolve by changing the downstream details of gene expression but not the master regulatory switches.