Difference between revisions of "Complementation"
(→Hereditary deafness) |
(→Complementation tables) |
||
| Line 207: | Line 207: | ||
*Gene 2 has mutant alleles 2 and 4. | *Gene 2 has mutant alleles 2 and 4. | ||
*Gene 3 has mutant allele 5. | *Gene 3 has mutant allele 5. | ||
| + | |||
| + | The table can be rearranged to make the complementation groups easier to visualize. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! | ||
| + | ! mutant 1 | ||
| + | ! mutant 3 | ||
| + | ! mutant 6 | ||
| + | ! mutant 2 | ||
| + | ! mutant 4 | ||
| + | ! mutant 5 | ||
| + | |- | ||
| + | ! scope="row" | mutant 1 | ||
| + | | style="background: red; color: white;" | - | ||
| + | | style="background: red; color: white;" | - | ||
| + | | style="background: red; color: white;" | - | ||
| + | | + | ||
| + | | + | ||
| + | | + | ||
| + | |- | ||
| + | ! scope="row" | mutant 3 | ||
| + | | style="background: red; color: white;" | - | ||
| + | | style="background: red; color: white;" | - | ||
| + | | style="background: red; color: white;" | - | ||
| + | | + | ||
| + | | + | ||
| + | | + | ||
| + | |- | ||
| + | ! scope="row" | mutant 6 | ||
| + | | style="background: red; color: white;" | - | ||
| + | | style="background: red; color: white;" | - | ||
| + | | style="background: red; color: white;" | - | ||
| + | | + | ||
| + | | + | ||
| + | | + | ||
| + | |- | ||
| + | ! scope="row" | mutant 2 | ||
| + | | + | ||
| + | | + | ||
| + | | + | ||
| + | | style="background: blue; color: white;" | - | ||
| + | | style="background: blue; color: white;" |- | ||
| + | | + | ||
| + | |- | ||
| + | ! scope="row" | mutant 4 | ||
| + | | + | ||
| + | | + | ||
| + | | + | ||
| + | | style="background: blue; color: white;" |- | ||
| + | | style="background: blue; color: white;" |- | ||
| + | | + | ||
| + | |- | ||
| + | ! scope="row" | mutant 5 | ||
| + | | + | ||
| + | | + | ||
| + | | + | ||
| + | | + | ||
| + | | + | ||
| + | | style="background: green; color: white;" |- | ||
| + | |} | ||
=Exceptions= | =Exceptions= | ||
Revision as of 08:02, 14 October 2017
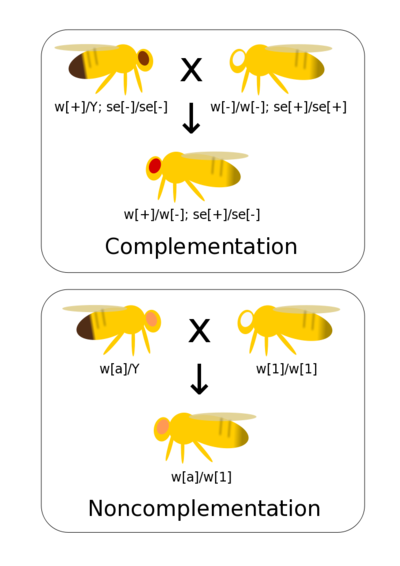
Complementation tests if two different alleles that result in observable phenotypes are part of the same gene or are alleles of different genes. If you have generated a range of mutants phenotypes for a trait it allows these mutations to be quickly grouped by genes (rather than, for example, conducting fine scaled recombinant mapping). Complementation assumes simple dominance where the mutant phenotype is recessive to wild-type.
If there are mutations in the same gene then when homozygous lines are crossed together there is not a wild-type allele present in the heterozygote. Thus, the heterozygote has a mutant phenotype. This is non-complementation and indicates that the two mutants occur at the same gene.
If there are mutations at two different genes then crossing true-breeding (homozygous) lines together generates double heterozygote offspring with a wild-type phenotype. This is complementation and indicates the two mutations occur at different genes.
Contents
Examples
Abstract example
Say we have three mutations, a[-], b[-], and c[-], that result in mutant wing phenotypes in Drosophila when homozygous. These mutant phenotypes are recessive to wild-type. First we cross a[-] and b[-] together.
a-/a-, c+/c+ x b-/b-, c+/c+
↓
a-/b-, c+/c+
a[-] and b[-] are two different alleles of the same gene and the wild-type phenotype is not restored (they failed to compliment). We can rename these as the a[1] and a[2] alleles.
Now we cross a[1] and c[-] together.
a[1]/a[1], c+/c+ x a+/a+, c-/c-
↓
a[1]/a+, c+/c-
This double heterozygote has a wild-type phenotype because the phenotype corresponding to a[1] and the phenotype corresponding to c- are both recessive to wild-type. We can infer that these are alleles of two different genes because they complemented each other (restored a wild-type phenotype). We rename c- the c[1] allele now that we know it is an allele of a different gene.
Examples
Classical genetic
You have a collection of eye color phenotypes that vary from wild-type red. You want to see if these are due to alleles of the same gene or of different genes. All of the mutant phenotypes are recessive to wild-type.
First you cross sepia and white together. The offspring are wild-type. You can infer that the offspring are double heterozygotes and that sepia and white are different genes.
Next you cross apricot and white together. The offspring are apricot, not wild-type. You infer that apricot is an allele of white.
Hereditary deafness
Deaf couples form more often than expected by random chance. This is an example of positive assortative mating in humans (and it has been suggested that this helps promote the transmission of sign languages, Aoki and Feldman 1991). However, heredity deafness follows a predominantly autosomal recessive inheritance pattern and there is a great deal of genetic heterogeneity (many different genes are often responsible, Christina M. Sloan-Heggen et al. 2016). Therefore, the offspring of deaf couples are often not deaf because of complementation. This has raised an ethical dilemma; some deaf parents choose to have children that are deaf with PGD (preimplantation genetic diagnosis) screening (Sanghavi 2006).
For a rough illustration the genes most often associated (a frequency of >1% in the total sample) with autosomal recessive deafness in Christina M. Sloan-Heggen et al. 2016 are listed in the table below (accounting for 93% of cases).
| Gene | Frequency | Frequency2 |
|---|---|---|
| GJB2 | 0.253 | 0.0640 |
| STRC | 0.191 | 0.0365 |
| SLC26A4 | 0.078 | 0.00608 |
| MYO15A | 0.056 | 0.00314 |
| MYO7A | 0.051 | 0.00260 |
| USH2A | 0.051 | 0.00260 |
| CDH23 | 0.048 | 0.00230 |
| ADCRV1 | 0.032 | 0.00102 |
| PCDH15 | 0.024 | 0.000576 |
| OTOF | 0.024 | 0.000576 |
| TMPRSS3 | 0.024 | 0.000576 |
| TECTA | 0.022 | 0.000484 |
| TMC1 | 0.022 | 0.000484 |
| LOXHD1 | 0.022 | 0.000484 |
| OTOA | 0.022 | 0.000484 |
| PTPRQ | 0.011 | 0.000121 |
| Sum | 0.931 | 0.122 |
Following the simplest assumptions (ignoring, for example, differences in allele frequencies between populations and assuming these frequencies represent homozygous genotypes for a causative allele) the probability that autosomal recessive deaf parents will both have the same gene responsible is the square of the genotype frequency (this uses the independence/multiplication rule of probability). One minus the sum of these squares is a rough approximation of the rate of complementation (non-deaf children due to autosomal recessive hereditary deafness), which works out to greater than 85% (1 - 0.122 = 0.878).
Deletion mapping
Lines with heterozygous deletions with well defined boundaries exist for some model organisms such as the Drosophila DrosDel collection (using the FLP recombinase system with FRT sites inserted across the genome by P-elements). If the deletion overlaps a mutation a mutant phenotype results from the hemizygote—the deletion and mutation are in non-complementation.
Larger deletions can be used to quickly find a region of the chromosome that the disrupted gene is located within. Then finer scale deletions within the larger region can be used to more precisely map the mutation.
(add example)
Heterokaryons
Two different nuclei of two cells can be artificially combined into a single multinucleate cell known as a heterokaryon (there are also examples of natural heterokayons). This allows the nuclei to share gene products within the cell and thus allows complementation testing between variants present in the parent cells.
(add examples, XP and chicken pigmentation)
Complementation tables
Pairwise crosses can be made and organized into a complementation table where a + indicates complementation (a wild-type phenotype in the offspring) and a - indicates non-complementation (a mutant phenotype).
| mutant 1 | mutant 2 | mutant 3 | mutant 4 | mutant 5 | mutant 6 | |
|---|---|---|---|---|---|---|
| mutant 1 | - | + | - | + | + | - |
| mutant 2 | + | - | + | - | + | + |
| mutant 3 | - | + | - | + | + | - |
| mutant 4 | + | - | + | - | + | + |
| mutant 5 | + | + | + | + | - | + |
| mutant 6 | - | + | - | + | + | - |
In the example table above mutants 1, 3, and 6 form a complementation group (fail to complement each other). Mutants 2 and 4 also form a complementation group. The mutants in this table represent allele at three different genes.
- Gene 1 has mutant alleles 1, 3, and 6.
- Gene 2 has mutant alleles 2 and 4.
- Gene 3 has mutant allele 5.
The table can be rearranged to make the complementation groups easier to visualize.
| mutant 1 | mutant 3 | mutant 6 | mutant 2 | mutant 4 | mutant 5 | |
|---|---|---|---|---|---|---|
| mutant 1 | - | - | - | + | + | + |
| mutant 3 | - | - | - | + | + | + |
| mutant 6 | - | - | - | + | + | + |
| mutant 2 | + | + | + | - | - | + |
| mutant 4 | + | + | + | - | - | + |
| mutant 5 | + | + | + | + | + | - |
Exceptions
Exceptions (except for dominant mutant phenotypes) are rare but they can give insights into gene products with functions that are closely associated.
Dominance
Cases where the mutant phenotype is dominant to wild-type, such as gain-of-function hypermorphs violate the assumptions used in complementation testing.
Example
(agouti vy and - amorph)
Intragenic complementation
If mutations that disrupt alternative promoters or alternative splicing variants are responsible for the altered phenotype then having two mutant alleles that are disrupted in different ways can restore a wild-type phenotype.
Example
(need to add example)
Unlinked noncomplementation
If gene products from two different genes directly interact, such as subunits in a complex, or their functions are closely associated, such as steps in the same biochemical pathway, they can fail to complement in a double heterozygote.
Example
(need to add example)
Links
http://www.wormbook.org/chapters/www_complementation/complementation.html
http://www.genetics.org/content/174/1/5